Partial Pressure
In the previous example the total pressure inside the "bomb" was 7 atm. We calculated that the pressures of the CO2 and the O2 were 3 and 4 atm respectively. The pressures of CO2 and the O2 are called partial pressures, because the pressures of the CO2 and the O2 add up to the total pressure.
Interestingly, the partial pressure of a gas is related to its
mole
fraction .
The total pressure is the result of the sum of all the gas molecules
present-the identity of the gases is not important just the number of
gas molecules.
Distribute the RT into the (na + nb).
Since
![]()
![]()
A balloon is filled with air at a pressure of 2 atm. Air is actually a mixture of gases, approximately 80% nitrogen and 20 % oxygen (by volume).
What is the partial pressure of the nitrogen in the balloon?
NOT 2 atm....how come?
Because the total pressure is 2.0 atm, and the total pressure is the sum of the partial pressures.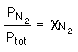
Collecting gases over water
Often a gas produced by a reaction can be collected over water; that is, a gas can be used to displace the water from and inverted container of water.
A graduated cylinder was filled with water and inverted in a tub of 22 °C water. H2 produced from the reaction of Zn with HCl was captured in the inverted graduated cylinder. With the water level inside and outside of the cylinder at the same level the volume of the gas was 90.0 mL. The barometric pressure was 761 torr. How many moles H2 were collected?
To determine moles of gas we need to know P, V, and T...
T = 22 °C = 295 K
P = ?
Level inside being equal to the level outside means the pressure inside is the same as the pressure outside...
This is the pressure of what? Is 761 torr the pressure of the H2 which was collected?
Wait...why not...
What gases are present in the graduated cylinder?
H2 is present, but so is H2O!
H2O evaporates, right?
So, the H2O in the cylinder will evaporate.
So, there is H2O vapor mixed with the H2.
Therefore,
What is the pressure of the water vapor? You can look this up! At 22 °C the pressure of the water vapor in equilibrium with the liquid water is 21 torr.
![]()
Now that the pressure of the H2 is known, the problem is just a PV = nRT problem...
solve for n