Gas Stoichiometry
We cannot count molecules so instead we weigh them;
however, it is extremely inconvenient to weigh gases. So, when adding
gases to a reaction how do we measure the amount of gas? We use the
Ideal Gas Law. How....
34.0 mL of a 6.0 M sulfuric acid solution is spilled on the floor. The acid is neutralized by pouring sodium hydrogen carbonate on the spilled acid. What is the volume, in L, of the carbon dioxide which is released? The gas being released is at 25 °C and 1 atm.
![]()
We need to know the number of moles of CO2 which are released to find the volume.
So, how many moles of acid are neutralized..
which is how many liters?
![]()
V = 9.98 L CO2
So, when gases are involved in reactions we can relate volume or pressure to moles using the Ideal Gas Law.
At constant temperature and presure, volumes of gas can be related directly to each other.
e.g.
At constant temperature and pressure 2 L of H2 are combined with 3 L of Cl2. How many liters of HCl will form?
Without knowing the temperaute and pressure we cannot determine the number of moles of either H2 or Cl2 present. Since the temperature and pressure are contant we can relate volumes of gas as though they are moles of gas....watch.
So,
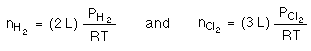
Normaly, to relate H2 to Cl2 we must convert to moles...
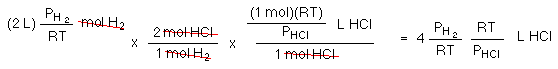
Since temperature and pressure are constant the pressure of H2 equals the pressure of HCl; so, the numbers needed to perform the conversion cancel out!
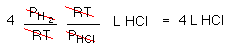
There is enough H2 to produce 4 L of HCl, but what about the Cl2?
This is really a limiting reagent problem hidden in a gas problem! There is enough Cl2 to make 6 L HCl, but there is only enough H2 to make 4 L of HCl.
"At constant temperature and presure volumes of gas can be related directly to each other."
A similar statement can be made about pressure and moles!
At constant temperature and volume the pressure of gases can be related directly to eachother.
The combustion of methane, CH4 at a pressure of 3 atm, in the presence of O2, at a pressure of 10 atm, occured in a sealed container. Determine the pressure of the gases in the container after the container returns to room temperature.
There is enough methane to make
There is enough oxygen to make
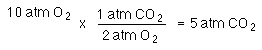
So, 3 atm CO2 will form. All of the CH4 will be consumed, but
leaving 1 atm of O2.
So, the total pressure is
![]()
P = 7 atm
This brings us to the concept of partial pressure.