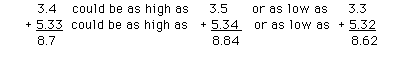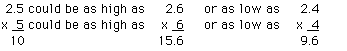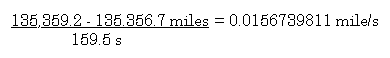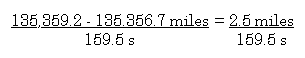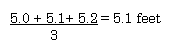Measurement (not
really much to say here)
1. Pick the right device.
2. Read the scale
a. digital .....read the scale
b. analog: read the value and then estimate last digit.
Generally one can estimate to 10% of the smallest scale
increment.
Since the last digit is estimated there is error associated
with it. Unless stated otherwise the error is assumed to be
±1 (the smallest increment).
e.g.
3.45 g means 3.45 ± 0.01 g
79.5 g means 79.5 ± 0.1 g
Reporting measurements
1. Units: do not forget the units. Additionally, include
names when appropriate.
do not simply write 3.19 g, but 3.19 g CuSO4
2. Significant Figures tell how precisely the measurement
was made.
A student uses a 4 decimal place balance to determine the
mass of a sample of NaCl. Should the mass be reported as 5 g, or
5.0000 g?
5.0000 g is the correct answer. If one were to write 5 g of
NaCl the assumption is that the error is ±1 g when the actual
measurement is precise to ±0.0001 g.
Using Significant figures
Two important steps with sigfigs...
1. Determining the
number of significant figures in a measurement.
a. All non-zero numbers are significant.
b. Zero's are significant if
i. they are between two non-zero numbers
ii. they occur before and after the decimal point of a
number whose absolute value is > 1
e.g. in 10.0 the zeros are significant.
iii. they occur after a non-zero number in a number whose
absolute value is < 1.
e.g. in 0.0560 the leading zeros are not
significant, but the trailing zero is.
2. Determining the number of significant figures that should
remain after performing mathematical manipulations with the
numbers.
There are two sets of rules
a. addition/subtraction
the result of adding two numbers together should be precise to
the place that corresponds to the least precise measurement
b. multiplication/division
the result of multiplying two numbers should have the same
number of significant figures as the number with the fewest
significant figures.
examples
a. Addition/subtraction
The measuments 3.4 in and 5.33 in could be as high as 3.5 and
5.34, or as low as 3.3 and 5.32
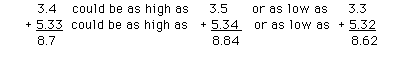
An answer of 8.73 would mean that the sum of the measurements
could be 8.74 to 8.72. Obviously, the sum can be outside this
small range due to the error associated with the measurement 3.4.
To ensure that the result falls within the real window of
uncertainty (i.e. 8.84 to 8.62) the result is reported as 8.7
in.
b. Multiplication (Determine the area of a rectangle with
side lengths equal to 2.5 and 5 cm)
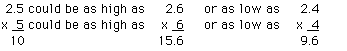
Once again using to many significant figures in the answer
would be misleading. The product of 5 and 2.5 is 12.5, but this
number implies a range from 12.4 to 12.6 cm2. Clearly,
the actual area could be outside the range of 12.4 to 12.6
cm2. 12.5 can rounded to 13. The result "13" implies
from 12 to 14 the actual possibilities are still outside this
range. To ensure that the result does not imply more precision
than is warranted, the result must be reported as 10
cm2, which implies that the number could be anywhere
from 0 to 20 cm2.
Combining Mathematical
operations
Losing Sgnificant Figures (lets demonstrate
with an example)
Because you are on a long automobile trip and you are
bored, you decide to determine your average velocity in miles per
second. The only instruments available are your odometer, and your
wrist watch.
You start the timer on your wrist watch when the odemeter reads
135,356.7 miles (did I mention that you are driving an old car?)
and you stop the timer when the odometer reads 135,359.2 mile.
Your stopwatch indicates that 159.5 seconds have elapsed.
Average speed equals distance traveled divided by
elapsed time.
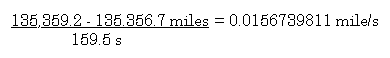
So, how many significant figures should be used in your
answer?
2
Why?
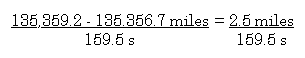
The difference is 2.5 and this number is the number that limits
the number of significant figures the answer can
contain.....so
0.016 mile/s (Incidentally, you are
travelling 56 mph.)
Exact
Numbers
Some numbers have an unlimited number of significant figures.
These numbers are called exact numbers. These numbers are
numbers about which one is absolutely certain.
•Exact numbers never limit the number of significant
figures.
•Exact numbers have as many significant figures as the
problem requires.
•Exact numbers are numbers that are defined to be a
certain value.
For example
12 inches = 1 foot
5280 feet = 1 mile
2.54 cm = 1 inch
1 km = 1000 m
•Exact numbers are things that can be counted. (This
statement must be applied cautiously. Every several years the
United States attempts to count the number of people in the
country. Clearly the population determined by the Census Bureau is
not an exact number.)
An Example
Three measurements were made of the length of my office.
5.0 feet, 5.1 feet, and 5.2 feet. Determine the average
length.
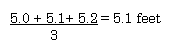
5.1 feet is correct. The "3" is an exact number so it does not
limit the number of significant figures contained in the
result.
An alternate view point... the answer should have three
significant figures.
Since 5.0 + 5.1 + 5.2 = 15.3 and 15.3 (following the
sigfig rules for addition produces a number with three sig-figs)
has three significant figures, the answer should also have three
significant figures. (return
to conversions page)
back to GenChem Homepage