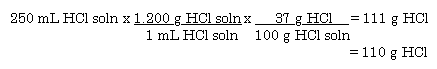How many centimeters in 2.00 miles?proportions
solve for x...
x = 10560 feetnext a proportion to switch to inches...
next a proportion to switch to cm... but if I did it this way I would grow old and gray so...
Conversions (To do chemistry problems you must be able to do conversions quickly.)
two methods are generally used...
1. proportions2. factor label a.k.a. unit analysis
some people think
using proportions is easier...
How many centimeters in 2.00 miles?proportions
solve for x...
x = 10560 feetnext a proportion to switch to inches...
next a proportion to switch to cm... but if I did it this way I would grow old and gray so...
some people think
using the factor label method is easier
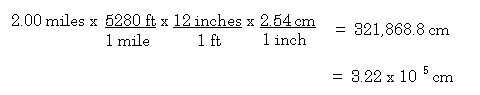
Why does factor label work? Well, all you are really doing is multiplying by 1....
What about significant figure here?
2.0, and 12 have 2 sig figs, and 1 has only 1 sig fig. So why does the answer have two sig figs?
The answer is that 1, 12, and 1 are exact numbers, and have as many significant figures as are needed.
Derived units are simply units created by combining other units like...
Density is g of material in 1 cm3 (or mL)% (percent) is number of parts of one constituent in 100 parts of the whole.
Percent is not enough information to make a valid unit. The unit of measure must be specified such as percent volume, or percent weight. (If only % is listed the unit is assumed to be % weight.)usually written % wt. and % vol.% by weight and % by volume are different.
ppm, ppb: parts per million, and parts per billion. These units mean number of particles per million particles.
Using derived units to perform conversions
For example, how much alcohol (in grams) is in a bottle of chardonay (0.750 L). A dry chardonay is typically labeled 13.5 % alcohol by volume.
So, we need to go from volume of wine to amount of alcohol in grams.....personally, I solve problems backwards....and then crunch numbers forwards.
Since we want grams of alcohol....(1st question) how can I get grams of alcohol from volume of wine? I can't, but I can take a smaller step; how about grams of alcohol from volume of alcohol. (alcohol d = 0.794 g/cm3).
grams of alcohol from density of alcohol and volume of alcohol
(be careful choosing the density to use you want grams of alcohol so you need to use density of alcohol, not density of wine, which inter converts grams of wine and volume of wine)(Did you notice that only one unit type is converted at a time? The conversion above is from volume of alcohol to mass of alcohol. The alcohol part of the unit stays the same.)
So, (2nd question) if we had volume of alcohol we could get grams of alcohol, but how will we get volume of alcohol?
volume of alcohol from % volume (of alcohol in wine) and volume of wine.
(Once again, only one unit type is converted at a time. % volume converts from volume of wine to volume of alcohol.)
To answer this question we converted between different types of measurements or units twice.
Notice that when I write units not only do I write the measurement unit, but I also write the name of the material to which the unit refers.
We need to know how much of the hydrochloric acid solution is HCl. The only information we have that relates amount of HCl to amount of solution is % wt. of HCl in the solution.
So, to find grams of HCl se need to know grams of solution.