|
|
|
|
|
|
|
|
|
|
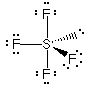
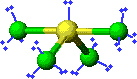
3D Model |
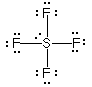
w/lone pairs |
|
|
 |
 |
|
|
|
|
 |
 |
|
|
|
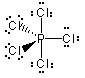
This example is the correct structure for SF4, but there is another possibility drawing which is the incorrect structure. compare |
||||
|
Why is this structure wrong? Lone pairs of electrons occupy more space than pairs of electrons that are in bonds. In this example, the lone pair of electrons bumps into bonding pairs at 90 ° angles in three directions. In the structure above, the lone pair of electrons bumps into bonding pairs at 90 ° in two directions. Thus, the repulsive forces experienced by the lone pair in the molecule above are lower than the repulsive forces experienced by the lone pair in the molecule on the right. |
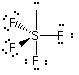 |
|
|
|

