The stoichiometry of the dissolution is called the vant Hoff factor. It is included in the expressions for freezing point depression and boiling point elevation as follows...
The van't Hoff factor is defined as follows:
The easy way to determine i.
For a substance that does not ionize or dissociate when added to the solvent i = 1; that is, for every mole of material dissolved one mole of solute is formed.
For substances that completely dissociate or ionize i = # of ions formed...

The van't Hoff number does not have to be a whole number
For substance that do not completely ionize like acetic acid in water the i value will not be an integer
Acetic acid dissolves in water to form aqueous acetic acid.
Once the acetic acid has dissolved some of it ionizes.
The reaction does not go to completion since acetic acid is a weak acid (we will discuss this in excruciating detail later). This means that two pariticles do not form for every one dissolved. "i" depends on the concentration of CH3CO2H and "i" is close to 1.05.
Additionally, aggregation can occur with compounds that ionize completely. In a concentrated solution it is possible that a Na+ and a Cl- ion can interact with each other. If the ions interact they can form ion pairs.
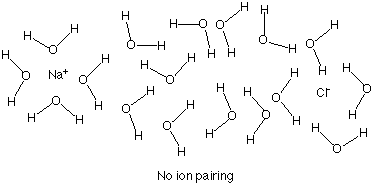
In dilute solution the ions are far appart, and
the ions do not interact with each other. i = 2.
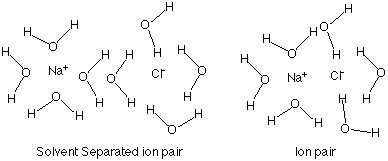
In a concentrated solution the ions are closer to each other, and the ions can interact with each other.When paired the ions act like 1 molecule not 2 ions. Thus 1 < i < 2
"i" could be less than one also... if a substance dissolves in a solvent and the substance aggregates in the solvent, then the number of particles dissolved in the solvent is lower than the number of molecules added to the solvent.
for example...
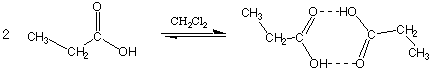
Propanoic acid forms H-bonded pairs when dissoled in solvents which do not H-bond. In this case i < 1.
van't Hoff factors in general
The van't Hoff factor for an ionic compound is the number of ions that form when the compound dissociates.The van't Hoff factor for a non-ionizing molecule is 1.
The vant Hoff factor for materials which are soluble in non-polar solvents is typically 1 (because these things are typically non-ionizing molecules).
The van't Hoff factor for an ionizing molecule, an acid, is the number of ions that form.