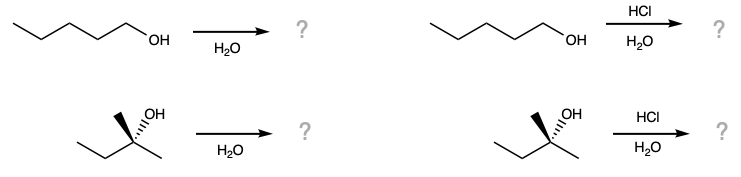
During this activity students will investigate the solubility of n- and t-pentanol in water and in aqueous hydrochloric acid. Students will also investigate the reactivity of t-pentanol with hydrochloric acid.
|
|
First, n-pentanol and t-pentanol are added to four test tubes. Note: The labels on the n-pentanol test tubes were erroneously reversed; that is, the aqueous HCl and water test labels should be switched. |
|
|
Before the water is added, make a prediction about whether you expect the alcohols to dissolve. |
|
|
Before the aqueous hydrochloric acid is added, make a prediction about whether you expect the alcohols to dissolve. |
|
|
t-Pentanol is added to a 15-mL centrifuge tube and the mass of the added t-pentanol is determined by comparing the mass of the empty container to the mass of the filled centrifuge tube. |
|
|
Hydrochloric acid is added to a centrifuge tube that contains t-pentanol. The centrifuge tube is shaken for 30 seconds, vented and schaken for an additional 3 minutes. TIP: The layers can be very difficult to see. Use a high contrast background to find the junction between the layers. Transferring the solution to a more transparent container may be helpful. Note in the video that the contrast between the dark line in the hood and the white background is the only thing that makes the transition from one layer to the next visible. |
|
|
Wash the product with 2 mL of water; that is, add 2 mL of water to the organic layer, shake, separate the layers, and discard the aqueous layer. TIP: The product, t-pentyl chloride, reacts with water, so this step should be done quickly. |
|
|
Add 2 mL of an aqueous 5% sodium bicarbonate solution to the organic layer. Shake, separate the layers, and discard the aqueous layer. TIP: The product, t-pentyl chloride, reacts with water, so this step should be done quickly. |
|
|
Several scoops of anhydrous sodium sulfate are added to dry the organic layer. TIP: The sodium sulfate should roll freely in the organic layer. If all of the sodium sulfate clumps together, add another scoop. |
|
|
The t-pentyl chloride can be separated from any t-pentanol that might remain by distilling the t-pentyl chloride. |
 The mass of the empty 3-mL conical vial. |
 The mass of the 3-mL conical vial and the t-pentyl chloride product. |
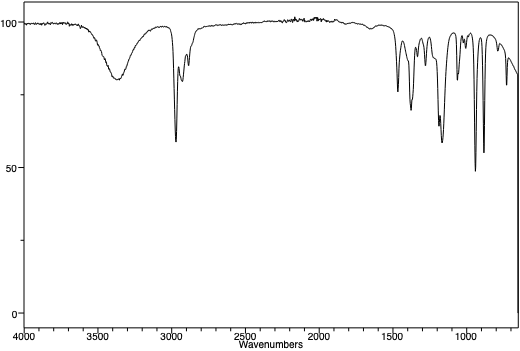 An infra-red spectrum of t-pentanol. Note the presence of the broad OH peak near 3450 cm-1. |
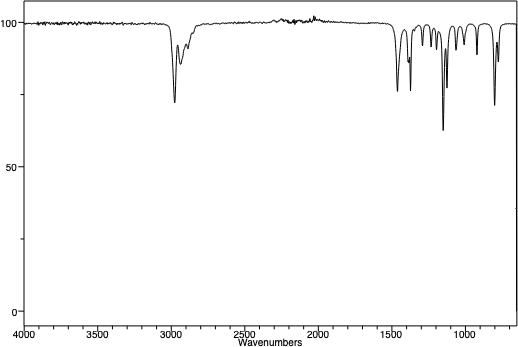 An infra-red spectrum of t-pentyl chloride. Note the absence of a broad OH peak around 3450 cm-1. Note the similarities between the three peaks in the two spectra at just below 3000 cm-1. This is the "C-H stretching region", and as the C-H framework of the molecule hasn't changed, one might expect that region of the IR spectra of the molecules to be similar. |