

During this activity, students investigate the regioselectivity of an electrophilic addition reaction. Students will carry out the reaction, purify the product by column chromatography, and perform tests on the product to determine whether the reaction successfully produced a bromohydrin and whether the hydroxyl group is at the secondary or tertiary position.
|
|
Carefully dispense 0.350 g N-bromosuccinimide (NBS) onto a piece of weighing paper. |
|
|
Add a spin vane, the tetrahydrofuran (0.75 mL), the water (1.0 mL), the NBS, and the 1-methylcyclohexene (0.24 mL) to a 5-mL conical vial and stir the contents of the vial. |
|
Add 2.0 mL of water to the reaction vial and separate the organic layer from the aqueous layer. Make certain to add the 2.0 mL of H2O. (The chemist forgot to add it while recording the video and forgot to make a second video when the reaction was repeated.) TIP: It is often easier to do the separation if you hold both vials next to each other in one hand while pipetting with the other. |
|
|
Confirm the identity of the organic layer by adding some water to the presumptive aqueous layer. Dry the organic layer with anhydrous sodium sulfate. Caution: Both the starting material and the organic solvent, THF, are less dense than water, but the brominated product is more dense than water. If too much THF or 1-methylcyclohexene were added, the organic layer will be less dense than the aqueous layer. On the other hand, when the organic solution has a high concentration of product dissolved in it, the organic layer will be more dense than water. |
|
|
Pack a pasteur pipet with silica gel and wet it with methylene chloride. Leaving the sodium sulfate behind, transfer the dried solution containing the organic product to the chromatography column. TIP: It is likely that you will not be able to add 3 mL of CH2Cl2 to the column all at once. |
|
|
Evaporate the CH2Cl2 from the product using a warm water bath and a gentle stream of compressed air. TIP: Make certain to adjust the flow of air to a gentle stream before it is positioned above the product solution.
|

An infrared spectrum of the product of the reaction. |
|
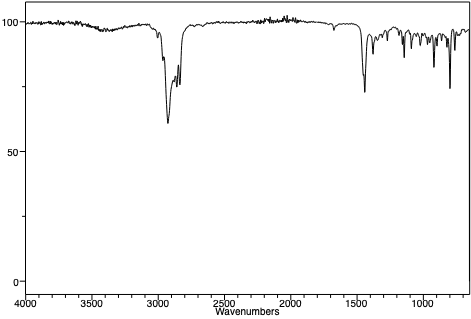
An infrared spectrum of 1-methylcyclohexene. |
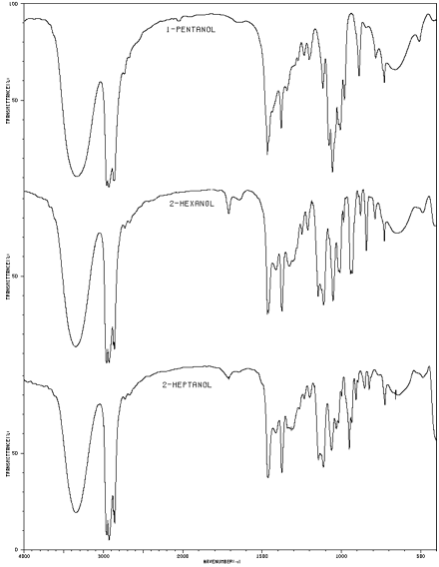
Infrared spectra of a 1-pentanol, 2-hexanol, and 2-heptanol. |
|

Infrared spectra of 1-pentene, 1-hexene, and 1-heptene. |
IR spectroscopic data for comparing known alcohols to alkenes of similar structure was obtained from the National Institute of Advanced Industrial Science and Technology, Japan.
http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
Silver Nitrate TestOn the left, a few drops of 2-bromobutane are added to a solution of silver nitrate disolved in ethanol. On the right, a few drops of the product from the electrophilic addition reaction are added to a solution of silver nitrate disolved in ethanol. |
|
Chromic Acid TestThe three test tubes are filled with 1 mL of acetone, and 1 drop of the chromic acid-sulfuric acid solution (the Jones reagent) is added to each test tube. On the left, drops of 2-methyl-2-propanol are added to the test tube. In the center, 2-propanol is added, and on the right, the product of the electrophilic addition reaction is added to the test tube. |