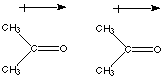
The negative end of one molecule (O) is attracted to the positive end (C) of the other molecule.
A dipole-dipole interaction is the attraction of the negative end of one permanent dipole to the positive end of another permanent dipole.
Last semester we learned how to determine if a molecule was polar. Two polar molecules can attract each other by the interaction of their dipoles. The more polar the molecule the stronger the interaction.The negative end of one molecule (O) is attracted to the positive end (C) of the other molecule.
|
|
|
|
A spce filling model of acetone. The different colors represent different elements. C is gray, H is white, and O is red. (The model may or may not be displayed when viewed with Internet Explorer.) |
A model displaying the charge distribution in an acetone molecule. The colors indicate the relative charges. The red color is the positively charged area of the molecule, and the blue color is the negatively charged area of the molecule. |