A molecule is polar if opposite charges exist on opposite sides of the molecule.
Step 1: Are there any polar bonds? If there are no polar bonds then the molecule cannot be polar.
Bonds are polar if two elements of differing electronegativities are bonded to each other. The more electronegative element pulls on the bonding electrons more strongly than the less electronegative element. Thus, there is an electron deficiency around the less electronegative element, a positive side, and there is a surplus of electron density around the more electronegative element, a negative side. The bond has a positive end and a negative end therefore, the bond is a polar bond.
Step 2: Do the polar bonds add up to a molecular dipole or do the dipoles cancel each other?
Determine the shape of the molecule using VSEPR rules, and check to see if dipoles oppose each other.
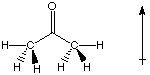
Acetone is a polar molecule because it has a polar bond, and the molecular structure does not cause the dipole to be canceled.
Step 1: Polar bonds?
C is slightly more electronegative than H (2.4 vs. 2.1).O is the second most electronegative element on the table. It is more electronegative than C (3.5 vs. 2.4). The C-O bond is polar.
Step 2: Resultant dipole?
There is no other dipole to cancel out the C-O dipole.
Conclusion: The molecule is polar.
|
|
|
|
A space filling model of acetone. The different colors represent different elements. C is gray, H is white, and O is red. (Since Internet Explorer does not full support Chime, the model does not always appear when viewed with Internet Explorer. Use Netscape 4.x instead.) |
A model displaying the charge distribution in an acetone molecule. The colors indicate the relative charges. The oxygen end of the molecule is a different color because it is more negatively charged. (Actually, this is a map of the nucleophilicity of acetone, which correlates reasonably well with charge distribution. The Java applet was having difficulty with the electrostatic potential map, but I am looking into this problem.) |