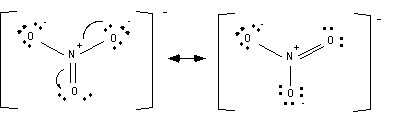
This structure brings up an interesting point.

If the electrons were arranged slightly differently the structure would look a bit different.
This is called resonance and it occurs when there is more than one valid lewis structure for a given molecule. It is important to note that no atoms move when a resonance structure is drawn. If an atom moves then what has been drawn is not a resonance structure; it is an isomer.
5. Write all the resonance structures for the molecule. Two resonance structures are drawn above; all of them are drawn below.
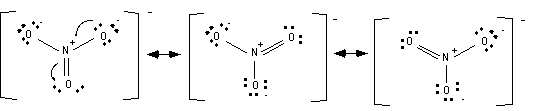
Since all these resonance structures are equal in energy the actual molecule is an average of the three, and the structure can be represented as...
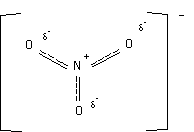
|
Dashed bonds are used to indicate partial bonds; however, because a popular chemical structure drawing program cannot draw dashed bonds often times curved solid lines will be used instead like in acetate... |
|
|
done |