|
C= 4 - (0 + 1/2 * 8) = 0 |
H = 1 - (0 + 1/2 * 2) = 0 |
Write the formal charge next to element (0 charge is omitted).
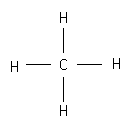
|
done |
4. Calculate the formal charge of each element.Formal Charge = (# e-'s element started with) - (# e-'s element ended up with)
Carbon started with 4 e-'s.Now how many did it end up with.
(# e-'s element ended up with) = (# unshared e-'s) + (1/2 # bonding electrons)
|
C= 4 - (0 + 1/2 * 8) = 0 |
H = 1 - (0 + 1/2 * 2) = 0 |

|
done |