Ionic bonding
Ionic compounds are held together by coulombic attraction. If there is a negatively charged ion next to a positively charged ion, the two ions will attract each other. Coulomb's Law quantifies the interaction
Q is the numerical ion charge; i.e., +1, +2, +3... for the cation and -1, -2 -3... for the anion. The radius in nm is "r".
E = -8.37 x 10-19 J
for 1 interaction
how about a mole of interactions, in kJ
E = -504 kJ mol-1
To remove an electron from a mole gaseous sodium atoms 495 kJ are required. This is just the molar ionization energy of Na.
When an electron is added to a mole of gaseous chlorine atoms 348 kJ are released.
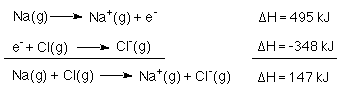
So, a chlorine atom cannot simply rip an electron of a Na atom. The attraction between the ions must make up for the uphill energy involved in abstracting the electron from the sodium atom. Afterall, the heat of formtaion of NaCl very large, DHf°= -411 kJ/mol.
The energy released by the attraction between the ions is called the Lattice Energy. The reaction is...
Experimentally determining the DH for the reaction written above cannot be done; however, Hess's Law can be used to calculate the energy involved when an ionic crystal is formed from gaseous ions.
If we can find an alternate path from NaF(s) to Na+(g) and F-(g) then we can use Hess's Law to dertemine the DHlattice. Step through the movie below using the frame advance button then continue reading.
Notice that the top of the diagram is Na+(g) and F(g). Hess's Law says that the energy required to go from the bottom state to the top state is the same regardless of path. So, the energy going up one side is the same as the energy going up the other side or..
Addtitionally, the energies must be compared for reactions that are all going in the same direction, so all the arrows must point in the same direction. Since we are saying the the energy required to go UP one side is the same as the amount of energy required to go UP the other side all the arrows must point up (if we had said down, then all the arrows have to point down). Since some reactions are being reversed; the corresponding DH's must also be reversed.
DHlattice = -923 kJ/mol
Which of the following two ionic solids will have a higher lattice energy?
|
Compare charges of the ions Q1Q2 = -2 |
Q1 = +1, Q2 = -1 Q1Q2 = -1 |
Mg2+ will be slightly smaller than Na+
Since the anions are the same, the "r" for MgF2 is slightly smaller than the "r" for NaF
Due to the charge of the ions the energy released by the coulombic attraction is more than twice as large for MgF2 than it is for NaF. So, the lattice energy for MgF2 should be more negative than the lattice energy for NaF.