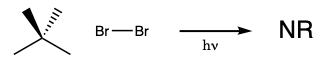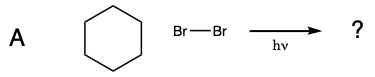
During this activity students will investigate the light-induced radical substitution reaction that occurs between bromine and hydrocarbons. Students will examine how the structure of several hydrocarbons affects their reactivity towards bromine.
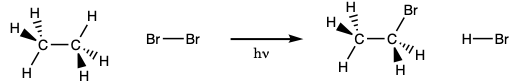
Light can initiate the reaction of bromine with hydrocarbons. The reaction of ethane with bromine drawn below is a hypothetical example of the reaction.

The substitution reactions occur through radical intermediates. During the initiation step of the reaction, the bond between two bromine atoms is broken homolytically. When this happens two bromine radicals form. In a propagation step, those bromine radicals can react with a hydrocarbon forming HBr and a hydrocarbon radical. In a second propagation step, the hydrocarbon radical can react with a bromine molecule forming a bromohydrocarbon and a bromine radical. Because the reaction of a radical, an odd electron species, and a non-radical, an even electron species, must form a radical as a product the propagation steps amount to a radical chain reaction that continues until two radicals just happen to react and form a non-radical. When two radicals come together in this way the reaction is referred to as a termination step because the formation of a non-radical from two radicals terminates the chain reaction. A schematic representation of the chain reaction appears below.
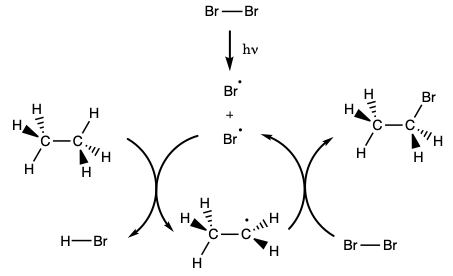
|
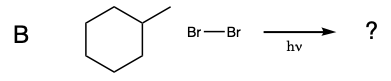
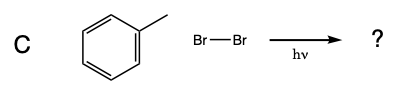
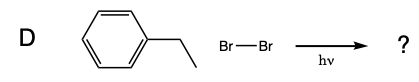
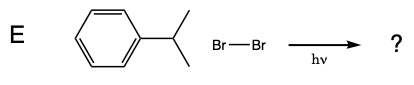
Five test tubes labeled A trhough E are placed in a beaker of water, and 1 mL of 0.08 M Br2 disolved in dichloromethane was added to each of the test tubes. In turn, a solution of cyclohexane in dicholoromethane is added to test tube A, a solution of methylcyclohexane in dicholoromethane is added to test tube B, a solution of toluene in dicholoromethane is added to test tube C, a solution of ethylbenzene in dicholoromethane is added to test tube D, and a solution of isopropylbenzene in dicholoromethane is added to test tube E. A light is turned on and the solutions are monitored for 15 minutes.
|
|
|
After 15 minutes at room temperature, test tubes still containing bromine are transfered to a warm water bath (approximately 45 °C). The brightness of the light is increased, and the reactions are monitored until only one test tube contains unreacted bromine. During the reaction, the warm water bath is changed several times since a hot plate was not used. |
|
The following reactions were not attempted, but the results of these experiments, had they been done, would be helpful in determining the products formed during this activity.