|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
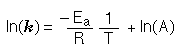
The activation energy can be determined by finding the rate constant of a reaction at several different temperatures.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
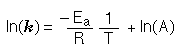
Notice that when the Arrhenius equation is rearranged as above it is a linear equation with the form y = mx + b; y is ln(k), x is 1/T, and m is -Ea/R. The activation energy for the reaction can be determined by finding the slope of the line.
|
|
Ea = -R•slope Which R? well what is the unit for Ea? Ea is in J/mol so are you going to use the constant 0.08206 L•atm•mol-1•K-1 or are you going to use the constant 8.314 J•mol-1•K-1. |
Ea = -8.314 J•mol-1•K-1(-9253 K)
Ea = 76929 ==> 77,000 J/mol
|
go back to 1st |