Instead of examining the vapor-liquid interface we look at the solid-liquid interface.
Boiling Point Elevation/Freezing Point Depression
Lowering the vapor pressure of a substance has an obvious effect on boiling point; the boiling point goes up. The BP increases because more energy is required for the solvent's vapor pressure to reach the external pressure.
The logic/picture which was used to explain vapor pressure lowering can be used to explain the change in boiling point. There are fewer solvent molecules at the surface capable of vaporizing so the vapor pressure drops. Thus, the temperature of the boiling point must be increased.
Another consequence of lowered vapor pressure is a decrease in freezing point. A picture can be used to explain this too.

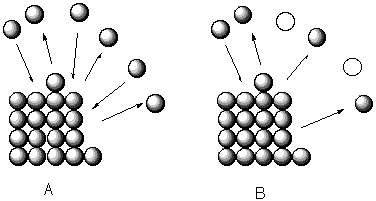
Instead of examining the vapor-liquid interface we look at the solid-liquid interface.
At a solid's normal melting point is defined for materials under 1 atm. of pressure as the temperature at which the solid is in equilibrium with the liquid.
At the freezing point the rate at which the solid melts is equal to the rate at which the liquid freezes A. If a solute is introduced the rate at which the solid melts does not change, but since there are fewer liquid molecules next to the solid the rate at which the liquid freezes drops--(B) three molecules melt and only one freezes--there is no longer an equilibrium and the solid melts. To reestablish the equilibrium the rate at which the solid melts must be lowered; i.e., the temperature must be lowered.
The change in both the freezing point and the boiling point is directly proportional to the amount of material dissolved in the solution
|
|
|
|
Because K is positive for both boiling point elevation and
freezing point depression the ![]() for BP elevation and the
for BP elevation and the ![]() for freezing point depression must be calculated differently.
for freezing point depression must be calculated differently.
A simple way to remember this is that the ![]() for the freezing depression is the amount the freezing point is
depressed (or lowered), and the
for the freezing depression is the amount the freezing point is
depressed (or lowered), and the ![]() for boiling point elevation is the amount the boiling point is
elevated (or raised). If you remember the direction then dealing with
the signs is easy.
for boiling point elevation is the amount the boiling point is
elevated (or raised). If you remember the direction then dealing with
the signs is easy.
It is important to note that Kb and Kf are constants for a given material, but vary from material to material.
Kf for water is 1.86 °C•kg•mol-1; whereas, Kf for benzene is 5.12 °C•kg•mol-1.