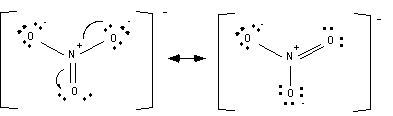
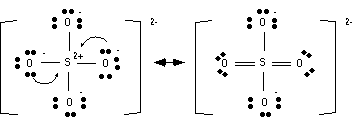
Structures that minimize formal charges are lower in energy than structures that have a lot of opposing charges. Remember only elements in the 3rd row or higher can accommodate more than 8 electrons.
Bonding pairs and lone pairs: since an orbital can hold two electrons we often talk about electrons in pairs. A bonding pair is the pair of electrons that are being shared. A lone pair is a pair of electrons that are not being shared.
First step: Draw the Lewis structures for each atom.
Distribute the valence electrons around the nucleus.Do not pair electrons until necessary.
Determining the placement of the atoms
The least frequently occurring element goes in the middle.
Formula's are written to help determine the structure. FCH3 does the F or the C go in the center of the molecule? The C atom. The formula FCH3 is written to show that the F and the H's are on either side of the C atom.Other hints....
First row elements will have a maximum of four bonds.
Carbon always has 4 bonds, of course, this is not strictly true, but for the molecules we talk about in General Chemistry it is.
Hydrogen always has 1 bond; once again, it is possible to have more than 1 bond to H, but you will have to go to graduate school to talk about molecules like this.
Nitrogen will usually have 3 bonds, occasionally 4; however, if the N has 4 bonds it will be positively charged. Nitrogen can also have 2 bonds if the nitrogen atom is negatively charged.
Oxygen will usually have 2 bonds, occasionally 3; however, if the O has 3 bonds it will be positively charged. Often, an oxygen atom will have only 1 bond, but the oxygen atom will be negatively charged.
Halogens usually have 1 bond; notable exceptions are polyatomic ions.
Second step: Circle unpaired electrons to indicate where bonds will form.
Continue circling unpaired electrons until all atoms have an octet of electrons.If you run out of unpaired electrons rearrange the electrons that remain and continue making bonds.
Third step: Redraw the molecule.
Replace circled electrons with lines indicating the presence of bonds.Make certain all remaining electrons are paired.
Fourth step: Determine formal charges and indicate the charge of the molecule.
Formal Charge = (# e-'s element started with) - (# e-'s element ended up with)
For the purpose of determining formal charge an atom "ends up with" all of its unshared electrons, and one half of the electrons in the bonds.
(# e-'s element ended up with) = (# unshared e-'s) + (1/2 # bonding electrons)
Fifth step: Determine resonance structures.
Resonance structures usually can be found by moving around electrons that are on atoms adjacent to double bonds.
Structures that minimize formal charges are lower in energy than structures that have a lot of opposing charges. Remember only elements in the 3rd row or higher can accommodate more than 8 electrons.