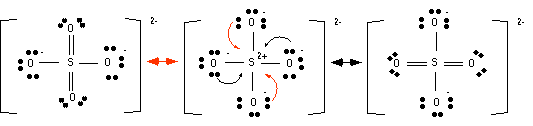
5. Normally one would say that no resonance structures can be drawn, but 3rd period elements are special. Remember 3rd period elements have d orbitals; they are just not filled. There is so much attraction between the S2+ and the O- that it attracts the electrons of the O-.

Now you say there are too many electrons around the sulfur...that is OK. The 3rd period elements can expand their octet by using the 3d orbitals. Also notice that the two structures on the left look similar, so the average structure looks more like the two on the left than the one on the right.
|
done |