|
N = 5 - (0 + 1/2 * 8) = 1 |
O = 6 - (6 + 1/2 * 2) = -1 |
|
H = 1 - (0 + 1/2 * 2) = 0 |
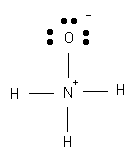
4. Determine formal charge.
|
N = 5 - (0 + 1/2 * 8) = 1 |
O = 6 - (6 + 1/2 * 2) = -1 |
|
H = 1 - (0 + 1/2 * 2) = 0 |

H3NO does not exist as a stable molecule, but a very similar compound, (CH3)3NO, does exist. Because there is a separation of + and - charge, (CH3)3NO is a very reactive molecule. (CH3)3NO is very good at adding one O atom to other compounds. (Can you draw (CH3)3NO ?)
|
done |