Molecular Orbital Theory
Goals: 5.1 Formation of Molecular Orbitals from Atomic Orbitals
5.1.1-5.1.4 Molecular orbitals from s, p, and d atomic orbitals
- Describe how s orbitals can interact to form molecular orbitals
- Draw the molecular orbitals that form from the interaction of two s orbitals
- Determine and explain the relative energies of the molecular orbitals that result from s-s interactions
- Describe how p orbitals can interact to form molecular orbitals
- Draw the molecular orbitals that form from the interaction of two p orbitals
- Determine and explain the relative energies of the molecular orbitals that result from p-p interactions
- Describe how d orbitals can interact to form molecular orbitals
- Draw the molecular orbitals that form from the interaction of two d orbitals
- Determine and explain the relative energies of the molecular orbitals that result from d-d interactions
Goals: 5.2.2 Homonuclear Diatomic Molecules
5.2.2 Orbital Mixing
- Describe how/explain why s orbitals can interact with p orbitals (orbital picture explanation)
- Explain what happens to the energy of the molecular orbitals when s/p mixing occurs
- Explain which p orbitals can and which cannot mix with s orbitals
5.2.3, 5.2.4 Diatomic Molecules of the First and Second Periods
- Draw MO diagrams for the diatomic molecules formed from the atoms of the first and second periods
- Draw molecular orbitals based on the starting orbitals and the g and u symmetry designations
- Comment about the information revealed by the molecular orbitals (bond order, magnetic properties, bond descriptions number of (
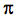 bonds)
bonds)
- Explain how the degree to which s and p orbitals mix can be determined
- Determine whether s/p orbital mixing has a significant effect on the energies of the molecular orbitals
- Determine the relative energies of the
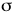 and
and 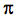 MO
MO
- Explain how photoelectron spectroscopy can be used to support molecular orbital diagrams
Goals: 5.3 Heteronuclear Diatomic Molecules
The MO diagram of HF and CO
- Draw MO diagrams for heteronuclear diatomic molecules
- Draw molecular orbitals based on the starting orbitals and the g and u symmetry designations
- Comment about the information revealed by the molecular orbitals (bond order, magnetic properties, bond descriptions number of (
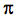 bonds)
bonds)
- Determine the relative contributions that the atomic orbitals make to the molecular orbitals (where the electrons are located)
Goals: 5.4.2 CO2
The Molecular orbitals of triatomic linear molecules
- Describe the basic reasoning behind forming group orbitals with peripheral atoms
- Using the appropriate character table, determine the reducible representation for the atomic orbitals that will be used to form the group orbitals for the atoms on the periphery
- Using the appropriate character table, determine the irreducible representations for the group orbitals formed from the atomic orbitals of the atoms at the periphery
- Determine which atomic orbitals of the central atom have the appropriate symmetry to interact with the group orbitals formed by the atoms at the periphery
- Draw a molecular orbital diagram that indicates which atomic and group orbitals are interacting to form the molecular orbitals---energy levels need not be placed perfectly, but careful consideration should be given to the the amount of interaction between the atomic orbitals and the group orbitals based on atomic orbital/group orbital energy differences
- Describe the nature of the molecular orbitals (bonding, nonbonding, antibonding), their location (evenly distributed over the atoms, primarily based on one atom, entirely based on one atom), and their appearance (for relatively simple molecular orbitals)
- Describe how/explain why s orbitals can interact with p orbitals (orbital symmetry explanation)
Goals: 5.4.4 NH3
- Using the appropriate character table, determine the reducible representation for the atomic orbitals that will be used to form the group orbitals for the atoms on the periphery
- Using the appropriate character table, determine the irreducible representations for the group orbitals formed from the atomic orbitals of the atoms at the periphery
- Determine which atomic orbitals of the central atom have the appropriate symmetry to interact with the group orbitals formed by the atoms at the periphery
- Draw a molecular orbital diagram that indicates which atomic and group orbitals are interacting to form the molecular orbitals---energy levels need not be placed perfectly, but careful consideration should be given to the the amount of interaction between the atomic orbitals and the group orbitals based on atomic orbital/group orbital energy differences
- Describe the nature of the molecular orbitals (bonding, nonbonding, antibonding), their location (evenly distributed over the atoms, primarily based on one atom, entirely based on one atom), and their appearance (for relatively simple molecular orbitals)
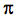 bonds)
bonds)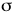 and
and 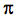 MO
MO